Original biological world
Written by Cong Wang
Edit Wang Duoyu
Typesetting and writing in water
Cell therapy is considered to be very suitable for treating Parkinson’s disease (PD) because its pathogenesis is relatively simple and characterized by localized degeneration of dopaminergic neurons in the substantia nigra compacta of the midbrain. In animal experiments and human experiments, transplanting human fetal ventral midbrain tissue or cells has been proved to help restore motor function.
However, these results are often inconsistent, which highlights the need for improved cell therapy programs to ensure that a sufficient number of well-defined midbrain dopaminergic (mDA) neurons are produced and standardized cell preparation and transplantation procedures are adopted.
Recent technological progress has enabled human pluripotent stem cells (hPSC), such as embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC), to efficiently generate mDA neurons, paving the way for establishing transplantation therapy for Parkinson’s disease based on hPSC. Many studies have shown that when transplanted into animal models of Parkinson’s disease, mDA progenitor cells derived from hPSC are well integrated into the host brain and mature into functional mDA neurons, which significantly improves motor function.
Recently, researchers from Yonsei University School of Medicine published a research paper entitled: preclinical and dose-ranging assessment of HESC-derived dopaminergic prognosticators for a clinical trial on Parkinson’s disease [1].
In this study, high-purity midbrain dopaminergic (mDA) progenitor cells were produced on a large scale from clinical human embryonic stem cells (hESC), and the safety and effectiveness of mDA progenitor cells were verified in vitro and in vivo. MDA progenitor cells improved the disease-related behavior of Parkinson’s disease rat model in a dose-dependent manner. Based on these preclinical research results, the research team obtained the approval of the Korean Ministry of Food and Drug Safety for the phase 1/2a clinical trial of cell therapy for Parkinson’s disease, and started the treatment of 12 patients with Parkinson’s disease.

In May, 2020, researchers at Massachusetts General Hospital reported a groundbreaking study in the New England Journal of Medicine (NEJM) [2]. A Parkinson’s patient who received autologous transplantation of mDA progenitor cells from hiPSC showed stable or improved clinical symptoms 24 months after implantation. Now, this method has been further developed, and the mDA progenitor cells derived from hiPSC and hESC began to enter the early clinical trial stage.
The first step in the clinical application of cell transplantation for Parkinson’s disease is to define the characteristics of cell products comprehensively. It is very important to determine whether cells are produced with clinical grade materials under GMP conditions and whether they meet the expected clinical use standards. Subsequently, preclinical research should produce convincing evidence of curative effect through large-scale animal experiments, and systematically examine long-term safety-related issues, such as toxicity, biological distribution and tumorigenicity. In addition, preclinical research needs to determine the optimal cell dose range for transplantation as a reference for human trials. Most importantly, the whole research design must be meticulous and the results must be evaluated in a fair way. This usually requires cooperation with CRO and extensive discussions with regulators.
This paper describes the process and results of a preclinical study aimed at using midbrain dopaminergic (mDA) neurons derived from human embryonic stem cells (hESC) in human clinical trials to treat Parkinson’s disease.
The cell differentiation method used in this study has been optimized for clinical application to ensure that it meets GMP standards. This optimization method can produce mDA progenitor cells with high purity and low temperature preservation on a large scale, while maintaining strict quality control. A year-long large-scale transplantation study by an independent CRO company using immunocompromised rats showed that the transplanted mDA neurons did not cause tumorigenicity, significant toxicity or ectopic integration outside the injection site. In addition, clinical mDA precursor cells showed therapeutic potential and dosage range, and produced therapeutic effects in toxin-induced semi-Parkinson’s rats. These findings provide the necessary information about the appropriate cell dose for human trials.
Specifically, this paper introduces the method of large-scale extraction of high-purity mDA progenitor cells from clinical grade hESC under strict GMP conditions, and also evaluates the toxicity, biological distribution and tumorigenicity of these cells in immunocompromised rats in facilities conforming to good laboratory practices (GLP). Different doses of mDA progenitor cells were transplanted into the semi-Parkinson’s rat model, and it was observed that there was a significant dose-dependent behavior improvement when the minimum effective dose ranged from 5000 to 10000 mDA progenitor cells. These results provide insights for determining the low cell dose (3.15 million cells) in human clinical trials.
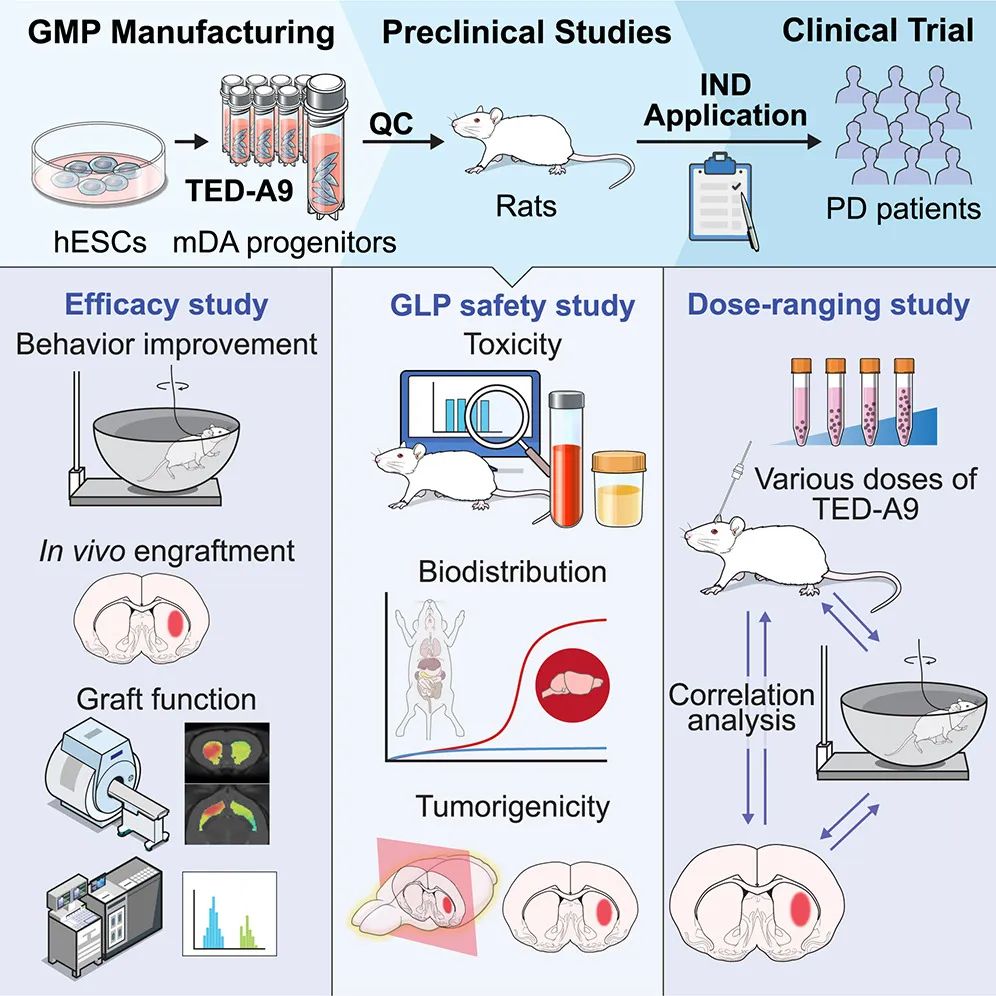
Based on these experimental results, the research team obtained the phase 1/2a clinical trial of cell therapy for Parkinson’s disease approved by the Korean Ministry of Food and Drug Safety, and started the clinical trial of treating patients with Parkinson’s disease.
Paper link:
1. https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(23)00401-0
2. https://www.nejm.org/doi/10.1056/NEJMoa1915872
Read the original text